 Mom of Linwood, ASMD
Mom of Linwood, ASMD
Tell us a bit about yourself and your family…
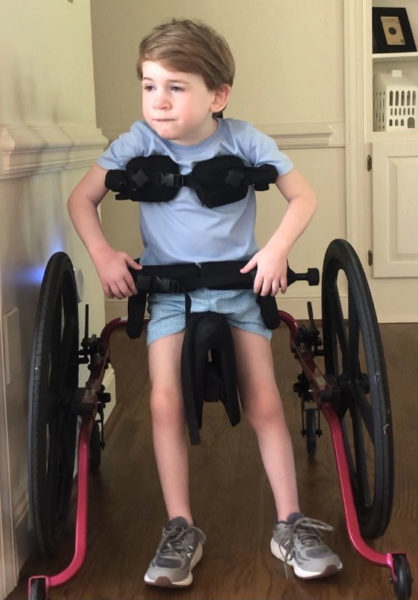
We are the Lewis’s!! We live in beautiful North Carolina, in the town where my husband, Lyn grew up. Tarboro is a small town like you see in the movies, where everyone knows everyone. We love spending time with our family and friends. Family is everything! Linwood is a busy five-year-old. He is in Pre-K and loves it. During the summer Linwood takes swim class, music class and has participated in therapeutic horseback riding. He loves being around his friends, and his cousin Hadley. When we are home Linwood enjoys watching movies with his dog, Major, and his kitten, Kitty Boop. Linwood also enjoys listening to music, especially Allan Jackson and Rudolph the Red Nose Reindeer. Linwood is our only child and is surrounded by so much love, he is the first boy on my side of the family in two generations!
 What led to and when did you receive the Niemann-Pick disease diagnosis?
What led to and when did you receive the Niemann-Pick disease diagnosis?
At thirteen months old, in 2017, Linwood went in for routine surgery, and the anesthesiologist discovered that his liver was enlarged. From there, Linwood was admitted to our local hospital, where he had multiple tests and labs done. From Leukemia to Lymphoma, and then to metabolic diseases, we were faced with many scary possibilities. As the test results came back, and the test being performed became more specific, we began to realize that Linwood had something very rare. The last test he had done was a liver biopsy. We were sent home after this test and were told we would be called with the results. At this point, the diagnosis was a metabolic disease, but it was between a glycogen storage disease and a lipid storage disease. We were told by the doctors that we did not want it to be a lipid storage disease. Linwood received his official diagnosis at fifteen months old, and it was Niemann-Pick type B.
What were the first steps you took after diagnosis?
We received Linwood’s diagnosis by his Pediatric Ophthalmologist during an eye exam. We had previously met with his genetic specialist, Dr. Spence at UNC, and the results of the liver biopsy had not come back yet. When we met with the pediatric ophthalmologist, she noticed the “cherry-red spot” at the back of Linwood’s eyes, which is an indicator of ASMD. So we asked the doctor if that meant Linwood had Niemann-Pick. She checked Linwood’s medical chart, and the results of the Liver biopsy were there, and it confirmed that he did have Niemann-Pick. After his diagnosis, we were informed by Linwood’s genetic specialist, Dr. Spence, that there was a clinical trial in New York and we were instructed to call because Linwood might qualify. We did what he said, and two weeks later we were making travel plans to fly to New York, for the screening process for the trial. After the screening process in February of 2018, we began in the trial two months later, when Linwood was eighteen months old.

How did you learn about NNPDF?
It was not until almost a year after Linwood’s diagnosis that my Stepmom actually invited me to join the NNPDF Facebook page. Up until this point, I honestly was afraid to join any support group or Facebook page, only because I was so afraid I would see something sad or scary. Our world had been turned upside down and we were still adjusting to our new normal. Our emotions were all over the place, and I knew if I saw something sad, that I wouldn’t be able to be strong for my child. Little did I know, NNPDF was exactly what we needed. It has been a blessing.
What caused you to get involved with NNPDF initially and how has being an NNPDF member benefitted your family?
After being a member for a while, Laurie Turner reached out to me. She asked if I would be interested in participating in a few projects with the NNPDF. After seeing so many brave families share their stories, and being able to lean on their comforting words, I was so honored to share Linwood’s story.

What impact has Neimann-Pick disease had on your life?
Niemann-Pick Type B/ASMD, has changed our lives. It has honestly opened our eyes to a world that we never knew existed. Prior to Linwood’s diagnosis, we had never heard of it. Even though there have been moments of uncertainty, fear, sadness, and a lot of worries, it has made us appreciate every single day, every single milestone, and every single thing that Linwood brings to our lives. I can honestly say it has made our lives more beautiful because we truly now understand how beautiful life is. I always knew that life was precious, but it wasn’t until we were faced with the unknown that we really understood.
What are your hopes for the future for yourself and for the Niemann-Pick community?
I pray that more research continues for those with Niemann-Pick, and I pray that with that comes more treatment options. I hope that those who are new to the NNPDF community, know that they are not alone, and even though they have their very own journey, they are not alone. I hope that awareness continues and that people get inspired to become involved. This is a wonderful community! Hopes for myself and my family, are that we continue to share our story, and hopefully are able to help others with doing so.
 Dear NPC Community Members,
Dear NPC Community Members,![]()





 This has been a historical week for the ASMD community. On March 28, Sanofi announced that olipudase alfa was approved in Japan for the treatment of pediatric and adult patients with ASMD! This is the first and only approved therapy for ASMD patients in the world! It took a village to help us reach this amazing milestone!
This has been a historical week for the ASMD community. On March 28, Sanofi announced that olipudase alfa was approved in Japan for the treatment of pediatric and adult patients with ASMD! This is the first and only approved therapy for ASMD patients in the world! It took a village to help us reach this amazing milestone!
 As members of the community may have heard, pharma company Orphazyme recently made the decision to withdraw its European Marketing Authorization Application (MAA) for arimoclomol for the treatment of Niemann-Pick disease Type C (NPC). According to a
As members of the community may have heard, pharma company Orphazyme recently made the decision to withdraw its European Marketing Authorization Application (MAA) for arimoclomol for the treatment of Niemann-Pick disease Type C (NPC). According to a 


 For people living with a rare disease, the road to diagnosis can be very difficult. Many patients must live through years of tests and doctor visits before they get the answers they need. This is especially common for people living with acid sphingomyelinase deficiency (ASMD) and the other types of Niemann-Pick disease.
For people living with a rare disease, the road to diagnosis can be very difficult. Many patients must live through years of tests and doctor visits before they get the answers they need. This is especially common for people living with acid sphingomyelinase deficiency (ASMD) and the other types of Niemann-Pick disease. 
 Each year for the past 15 years, the Niemann-Pick disease community has joined with patients, families, healthcare professionals and advocacy organizations around the world to recognize Rare Disease Day (RDD). On the last day of February, we take this special opportunity to show our support for our own community and for the over 300 million people affected by rare diseases. RDD is an opportunity to help more people learn about the impact of rare diseases and call for more research and programs that can make a positive difference. This year’s theme, “Share Your Colors”, reflects one of our core values at NNPDF – our commitment to elevating all of the voices in our community in an effort to build broader awareness of Niemann-Pick disease.
Each year for the past 15 years, the Niemann-Pick disease community has joined with patients, families, healthcare professionals and advocacy organizations around the world to recognize Rare Disease Day (RDD). On the last day of February, we take this special opportunity to show our support for our own community and for the over 300 million people affected by rare diseases. RDD is an opportunity to help more people learn about the impact of rare diseases and call for more research and programs that can make a positive difference. This year’s theme, “Share Your Colors”, reflects one of our core values at NNPDF – our commitment to elevating all of the voices in our community in an effort to build broader awareness of Niemann-Pick disease.
 Mom of Linwood, ASMD
Mom of Linwood, ASMD What led to and when did you receive the Niemann-Pick disease diagnosis?
What led to and when did you receive the Niemann-Pick disease diagnosis?



 As I look forward to the New Year, I am so encouraged by bravery and resilience of the many families that work with us. I believe our community is stronger than ever. Throughout 2021, we had some signs of progress, and we faced some setbacks when promising clinical development programs and trials were either cancelled or failed to win FDA approval. In response, we saw our community join together to fight even harder to address the issues that are important to us. I am inspired by so many examples of bravery and determination. Dale Carnegie once said, “Most of the important things in the world have been accomplished by people who have kept trying when there seemed to be no hope at all.” Our community is continuing to fight for our families despite our challenges. We are closer than ever to having approved treatments in Niemann-Pick disease. And together we will make a positive difference.
As I look forward to the New Year, I am so encouraged by bravery and resilience of the many families that work with us. I believe our community is stronger than ever. Throughout 2021, we had some signs of progress, and we faced some setbacks when promising clinical development programs and trials were either cancelled or failed to win FDA approval. In response, we saw our community join together to fight even harder to address the issues that are important to us. I am inspired by so many examples of bravery and determination. Dale Carnegie once said, “Most of the important things in the world have been accomplished by people who have kept trying when there seemed to be no hope at all.” Our community is continuing to fight for our families despite our challenges. We are closer than ever to having approved treatments in Niemann-Pick disease. And together we will make a positive difference.