Summer 2022 Newsletter
APPROVAL! Xenpozyme for Treatment of ASMD | Community Update Series: Xenpozyme
Message from the Board Chair | 2022 Family Support & Medical Conference Recap
NNPDF 2022 Awards | NNPDF 2022 Board of Directors | NNPDF Welcomes New Board Members
Duke-Margolis Center Webinar | ASMD Accelerate | AllStripes Update
ACT for ALS Workshop | NNPDF In Action | Clinical Trial Update
Fundraising | Membership | Emergency Hardship Program
APPROVAL!!
Xenpozyme (olipudase alfa) for Treatment of ASMD
We are pleased to share the U.S. Food and Drug Administration (FDA) has approved Xenpozyme™ (olipudase alfa-rpcp) for the treatment of non-central nervous system (non-CNS) manifestations of acid sphingomyelinase deficiency (ASMD) in adult and pediatric patients! Xenpozyme is the first therapy indicated specifically for the treatment of ASMD, and is currently the only approved treatment for this disease.
This has been a long road and the ASMD community played a vital and persistent role in bringing this medicine through the trials process and to an approval. We are incredibly pleased that we now have a first approved medicine in the Niemann-Pick community.
Community Update Series presents: Xenpozyme (olipudase alfa) update with Sanofi’s Patient Supports Services

Join us for a community meeting with Sanofi’s Patient Supports Services as they share more information regarding the approval of Xenpozyme treatment of ASMD.
Wednesday, September 7th, 2022
8:00 pm ET
Join Zoom Meeting
Message from the Board Chair

Dear, NNPDF Community,
As the conference came to a close this year, I was reminded of the strength of our community yet again. Our community gathered in-person and virtually during this year’s conference and I could not be prouder of our PERSEVERENCE through all that our community has faced in the past year. Our conference not only symbolized but brought to life the endurance and insurmountable strength of who we are when we are all together. We support each other and support our community. Thank you to all who participated, from our families, our clinicians, researchers, scientists, and sponsors.
Thank you for the incredible moments at our conference, from pointed questions, extraordinary feedback, love shared, siblings uniting, and dance moves like no one was watching. It was an honor hosting the INDPA. Partnership and strategy are at the forefront of everyone’s intentions and execution of initiatives. Thank you to all for the conference, it was not only informative for so many, but collaborative and just downright refreshing to see old friends and new.

The momentum from the conference has continued on in the work that we do. We have collaborated with industry and families to elevate our voices and we will keep our eyes on all that our families face and how we can help. My wish, as I start my tenure as Board Chair, is to continue the trajectory of the foundation with an incredible board and staff. Our voices and our community have come so far and we still have a long way to go, but I have faith that our efforts combined are going to impact the lives of our loved ones now and in the future.
The recordings will be available in the upcoming weeks. We hope that you were able to joyfully connect, meet new families, reconnect with friends, and truly live in this journey knowing that you are supported and loved by us all. We say it often, but it never loses its power, we may not be the family you ever wanted to have, but we are the family that will never leave your side. No matter where you are on this journey, your voice will be heard, you have us to lean on, and we remain founded in our roots. We believe deeply in the future of research that promotes clinical treatments with potential to improve patient quality of life and with intent to ultimately treat and cure all Niemann-Pick disease types.
Warm Regards,
![]()
Becky McGuire
NNPDF Board Chair
NNPDF Family Support & Medical Conference

THANK YOU to all that joined us in Orlando and virtually for the 30th Annual Family Support & Medical Conference. We were so happy to meet with you in person! New friendships and connections were made, we learned more about each other, and how we can help one another to grow as a community in the US and abroad.
In the upcoming days we will be sharing links to the LiveStream sessions and photos of the event.
Thank You to our Family Support & Medical Conference Sponsors
NNPDF Award Recipients
We are honored to present our 2022 award recipients! Thank you for the impact you have in the Niemann-Pick community and your chosen career paths. Congratulations!


Persevere Award
Dr. Ed Schuchman
NNPDF Cora Sterling
Endurance Award
Christopher Sousa


Joele Ruppert & Joseph Colton
ASMD Scholarship
Yasmin Markman
Joele Ruppert & Joseph Colton
ASMD Scholarship
Jack Visoky
NNPDF 2022 Board of Directors
We are honored to present your 2022 NNPDF Board of Directors. NNPDF Board members generously volunteer their time and energy to keep your family support organization moving forward and are essential in the progress of Niemann-Pick Disease awareness. Thank you to each of you for serving in these vitally important roles.
FRONT ROW: Becky McGuire (Board Chair), Paul Merrigan, Travis Obermeyer, Cara Gilmore, Anthony Leoni, and Kari Lato. BACK ROW: Joslyn Crowe (Executive Director), Gail Koujaian, Taylor Sabky, Mary Francis Harmon, Liz Heinze (Vice Chair), Meghann Ferguson (Secretary), and Mike Smith (Treasurer).
NNPDF Welcomes New Board Members
We are proud to welcome the following incoming Board Members to the NNPDF team: Gail Koujaian, Travis Obermeyer, and Taylor Sabky. Learn more about them.



Gail Koujaian
Mother of Alec,
and Hayley (In Memory), NPC
Travis Obermeyer
Father of Austin, ASMD
Taylor Sabky
Mother of Purnell
(In Memory), ASMD
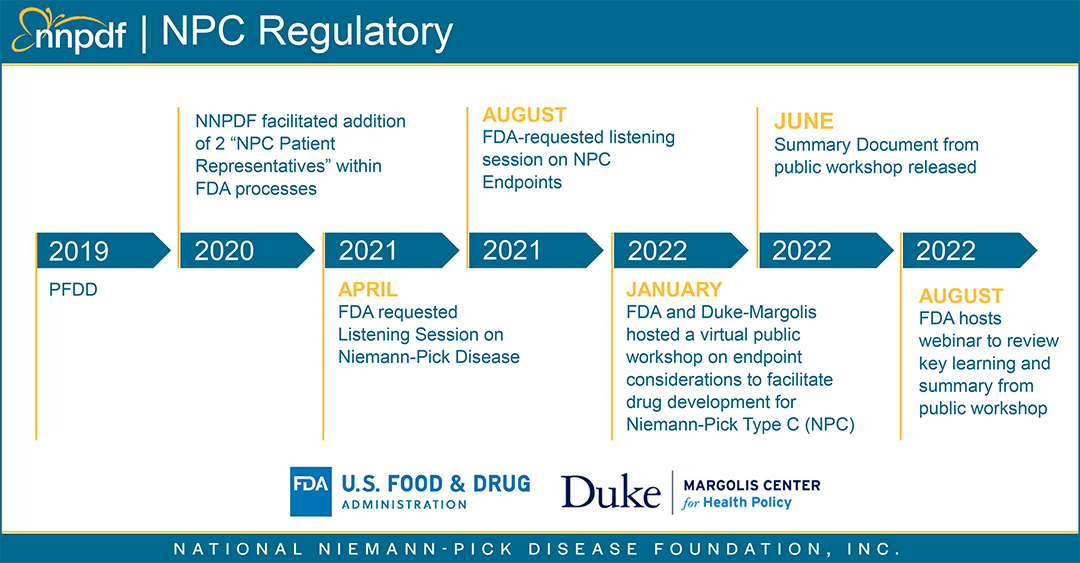
Duke-Margolis Center Webinar

On August 4th, the FDA and Duke Margolis Center for Health Policy held a webinar on Endpoint Considerations to Facilitate Drug Development for Niemann-Pick Type C which discussed key themes from the January 2022 public workshop. Discussion included a review of challenges, and opportunities for endpoint selections in NPC to support product development.
The recording for this webinar is available here.
This discussion was focused on a longer-term view of drug development including the importance of biomarkers and endpoints in NPC drug development. NNPDF recognizes the importance of these scientific areas and will continue to work in partnership with all NPC community organizations and foundations to assist with biomarker development. Our goal as a national patient organization is to ensure that we simultaneously continue to ensure attention and focus on our investigational therapies currently in development and continue to find paths to move to approved therapies expediently.
It’s not too late to join ASMD Accelerate!
Join ASMD Accelerate to support the ASMD research community and improve care! Thank you to the 15 families who have contributed their child’s de-identified medical data in support of ASMD research. We are 60% of the way to our goal and still enrolling for this incredibly important study. Your help can shape the future of ASMD care.
Signing up for ASMD Accelerate takes less than 10 minutes and PicnicHealth will do the hard work of collecting your child’s records on your behalf. Enroll today by visiting picnichealth.com/asmd-wylder-nation or email [email protected] with any questions.
AllStripes Update

AllStripes recently informed NPC families that with deep regret they will no longer be supporting the NPC Sibling Study. In response to broader economic downturn, AllStripes is reprioritizing its resources so that they may continue to sustainably support rare disease research. Unfortunately, this means that they cannot continue resourcing several studies, including the NPC sibling study.
 We (NNDPF, APRMF, Firefly Fund, and NP Canada) have chosen to partner with the International Niemann-Pick Disease Registry (INPDR) moving forward and to continue the NPC sibling study through the INPDR. The INPDR is the Niemann-Pick specific patient registry which is led and governed by the Niemann-Pick community. We are recommending that all NPC families who have shared their loved one’s data with AllStripes have their data transferred to INPDR. This is completely voluntary and each family’s decision is respected. We have worked with AllStripes and the INPDR to ensure that this is a user-friendly and fairly simple process for families.
We (NNDPF, APRMF, Firefly Fund, and NP Canada) have chosen to partner with the International Niemann-Pick Disease Registry (INPDR) moving forward and to continue the NPC sibling study through the INPDR. The INPDR is the Niemann-Pick specific patient registry which is led and governed by the Niemann-Pick community. We are recommending that all NPC families who have shared their loved one’s data with AllStripes have their data transferred to INPDR. This is completely voluntary and each family’s decision is respected. We have worked with AllStripes and the INPDR to ensure that this is a user-friendly and fairly simple process for families.
For information on how to share you loved one’s records with the INPDR, or if you have any questions, please contact us at [email protected].
Virtual Roundtable Discussion of the ACT for ALS Bill

In July 2022 NNPDF held a roundtable discussion on the Accelerating Access to Critical Treatments for ALS ACT, which is designed offer grants and other financial incentives to support expanded access programs in ALS and other rare neurodegenerative diseases. The forum included 15 U.S. organizations focused on rare neurodegenerative diseases.
A panel of experts provided an overview of the bill including the FDA Action Plan and the Grant Program.
Top findings include:
- A need to bring representatives from CDER, CBER, and other divisions at the FDA to establish consistency between Centers and form a predictable path for drug development.
- The option to consider alternatives to placebo trials in ALS.
- Calls for higher levels of funding.
- NIH Grant applications are open now for ALS with notification in Sept 2022.
NNPDF will continue to host these forums, our actions for next steps include:
- A series of quarterly discussions to continue to invite community perspectives on program execution.
- Updates on the logistics of the FDA Grants Program as they become available.
- Review of the benefits of a new alliance of organizations in rare neurodegenerative diseases to represent community interests with a stronger voice.
NNPDF In Action
 Evren Ayik, and Justin and Garrett Hopkin attend Sanofi’s 2022 TORCH Awards in Cambridge, Massachusetts.
Evren Ayik, and Justin and Garrett Hopkin attend Sanofi’s 2022 TORCH Awards in Cambridge, Massachusetts.


Clinical Trial Updates

Clinical trials are currently in progress to study and develop treatments for ASMD and NPC. The NNPDF posts new information regarding clinical trial updates as soon as it is received. Visit our Clinical Trials web page for up to date information on all clinical trials.
Update from Sanofi: We are pleased to share the U.S. Food and Drug Administration (FDA) has approved Xenpozyme™ (olipudase alfa-rpcp) for the treatment of non-central nervous system (non-CNS) manifestations of acid sphingomyelinase deficiency (ASMD) in adult and pediatric patients! Xenpozyme is the first therapy indicated specifically for the treatment of ASMD, and is currently the only approved treatment for this disease.
This has been a long road and the ASMD community played a vital and persistent role in bringing this medicine through the trials process and to an approval. We are incredibly pleased that we now have a first approved medicine in the Niemann-Pick community.
Read Sanofi press release.
Read FDA press release.
Read Xenpozyme press release.
Update from Sanofi: European Approval! News from Sanofi on Xenpozyme (olipudase alfa): “Xenpozyme® (olipudase alfa) approved by European Commission as first and only treatment for ASMD”. The European Commission (EC) has approved Xenpozyme® (olipudase alfa) as the first and only enzyme replacement therapy for the treatment of non-Central Nervous System (CNS) manifestations of Acid Sphingomyelinase Deficiency (ASMD) in pediatric and adult patients with ASMD type A/B or ASMD type B. Read the press release.
Surveys, Studies, & Market Research
 Be sure to check out our Surveys & Market Research webpage for current survey and study opportunities in the Niemann-Pick disease space. Participating in surveys and studies is important to the advancement of health options for our Niemann-Pick community members. Contact Laurie Turner at [email protected] for any questions.
Be sure to check out our Surveys & Market Research webpage for current survey and study opportunities in the Niemann-Pick disease space. Participating in surveys and studies is important to the advancement of health options for our Niemann-Pick community members. Contact Laurie Turner at [email protected] for any questions.
Fundraising
 Contributions through fundraising by NNPDF members, families and friends are used to provide services and information to individuals and families affected by NPD, as well as supporting research into finding treatments. Please continue to host and support NPD fundraisers. Awareness Events promote awareness to the general public about Niemann-Pick Disease.
Contributions through fundraising by NNPDF members, families and friends are used to provide services and information to individuals and families affected by NPD, as well as supporting research into finding treatments. Please continue to host and support NPD fundraisers. Awareness Events promote awareness to the general public about Niemann-Pick Disease.
If you have recently hosted a fundraising event, send us your photos and we’ll share them and details from your event in upcoming newsletters!

 THANK YOU to Stacy Stemmerman, Lane Hemsley, Stacy Tillotta, Vera Stricklin, and Natalie Johnson who recently hosted Facebook Fundraisers for the NNPDF! We truly appreciate your support!
THANK YOU to Stacy Stemmerman, Lane Hemsley, Stacy Tillotta, Vera Stricklin, and Natalie Johnson who recently hosted Facebook Fundraisers for the NNPDF! We truly appreciate your support!
Want to host your own Facebook Fundraiser? It’s easy! Visit facebook.com/fund/NNPDF to get started!
NNPDF Membership
 Enrolling, confirming, or updating your membership will ensure we have accurate information for you and your family. This will help us to continue to provide you with important notifications and updates from the NNPDF.
Enrolling, confirming, or updating your membership will ensure we have accurate information for you and your family. This will help us to continue to provide you with important notifications and updates from the NNPDF.
Click here to update or enroll today!
For assistance contact Laurie at [email protected] or call 920-542-4038
Emergency Hardship Program
 The NNPDF Emergency Hardship Program continues to offer assistance to qualified NNPDF U.S. member families facing a crisis. Funding includes but is not limited to, specialized medical equipment, durable medical goods, utility bills (heating and cooling, electricity, phone, water and sewer), home and car repairs, and bereavement expenses. Click here for complete details and eligibility information.
The NNPDF Emergency Hardship Program continues to offer assistance to qualified NNPDF U.S. member families facing a crisis. Funding includes but is not limited to, specialized medical equipment, durable medical goods, utility bills (heating and cooling, electricity, phone, water and sewer), home and car repairs, and bereavement expenses. Click here for complete details and eligibility information.
Please contact Laurie Turner, Family Services Manager at 920-542-4038 or [email protected] if you have any questions about this program.









