Niemann-Pick ASMD
Diagnosing ASMD (Types A and B)
Niemann-Pick Types A and B (NPA and NPB), also called Acid Sphingomyelinase Deficiency (ASMD), are caused by the deficiency of a specific enzyme, acid sphingomyelinase (ASM). This enzyme is found in special compartments within cells called lysosomes and is required to metabolize a lipid called sphingomyelin. If ASM is absent or not functioning properly, sphingomyelin cannot be metabolized properly and is accumulated within the cell, eventually causing cell death and the malfunction of major organ systems.
NPA and NPB are both caused by the same enzymatic deficiency and there is growing evidence that the two forms represent opposite ends of a continuum. People with NPA generally have little or no ASM production (less than 1% of normal) while those with NPB have approximately 10% of the normal level of ASM.
NPA as well as NPB, are diagnosed by measuring the level of activity of an enzyme called acid sphingomyelinase (ASM) in white blood cells. The test can be performed after taking a small blood sample from an individual suspected of having the disease and is available at many commercial laboratories in the United States and elsewhere.
While this test will identify persons with Type A (as well as Type B), it is not very reliable for detecting persons who are carriers (who have only one non-functional copy of the ASM gene). Further, the test will show decreased activity of ASM, but it cannot always predict whether the individual will have Type A or Type B or an intermediate variant of the disease; that requires clinical evaluation of the individual.
Molecular genetic testing is now available commercially for Niemann-Pick disease, type B (or ASM Deficiency) at several laboratories, including GeneDx in Gaithersburg, MD, Ambry Genetics in Aliso Viejo, CA and Emory Molecular Genetics Laboratory in Atlanta, GA. Your health care provider should contact laboratory personnel to arrange for testing if you are interested. Once an affected individual has been tested and his or her mutations have been identified, it is then possible to diagnose Type B carriers by DNA testing within the individual’s family.
The Mount Sinai Department of Human Genetics has identified certain populations* (shown below) where specific mutations account for a high percentage of cases of ASM Deficiency*. In these populations, it is possible to screen individuals for these specific mutations in order to identify carriers. In other populations, the mutations must first be identified in the affected individual before DNA carrier testing can be performed within a family, as noted above.
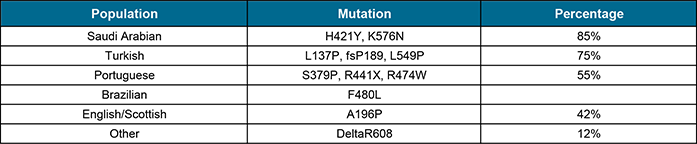
In other populations, the mutations must first be identified in the affected individual before DNA carrier testing can be performed for family members (by tradece potter). More recently, comprehensive analysis of the entire ASM gene structure has been used for carrier testing for partners of known Type A carriers. This is available at several US laboratories, including GeneDx in Gaithersburg, MD, Ambry Genetics in Aliso Viejo, CA and Emory Molecular Genetics Laboratory in Atlanta, GA.
For NPA, the mutations R496L, fsP330 and L302P account for over 95% of disease-causing genetic changes in the Ashkenazi Jewish population. Direct testing of individuals in this population for these 3 changes is used for carrier identification.
If you have any questions about diagnostic or molecular genetic testing for Niemann-Pick Disease, Type B or need assistance in arranging testing, contact the NNPDF.
*Information from “Identification and expression of a common missense mutation (L302P) in the acid sphingomyelinase gene of Ashkenazi Jewish type A Niemann-Pick disease patients” by Levran O, Desnick RJ, Schuchman EH. Blood, Oct 15, 1992.
Additional Information:
ASMD Clinical Guidelines
Acid Sphingomyelinase Deficiency NORD
A Practical Guide to Acid Sphingomyelinase Deficiency Niemann-Pick disease Type B
ASMDFacts.com
The Assistance Fund
Understanding ASMD: Information for You and Your Family Download Here
What is ASMD Niemann-Pick disease? Video published by NPUK
Contacts at:
Edward H. Schuchman, PhD
Genetic Disease Foundation – Francis Crick Professor of Genetics & Genomic Sciences
Vice Chair for Research, Department of Genetics & Genomic Sciences
Director, International Center for Types A & B Niemann-Pick Disease
Icahn School of Medicine at Mount Sinai
1425 Madison Avenue, Room 14-20A
New York, NY 10029
Tele: 212-659-6711; Fax: 212-849-2447
edward.schuchman@mssm.edu
George A. Diaz, MD, PhD
Division Chief of Medical Genetics
Mount Sinai School of Medicine
Atran Berg Laboratory Building, 1 – 45
1428 Madison Avenue
New York, NY 10029
Tele: 212-241-0858
george.diaz@mssm.edu
Brittany Keiser
Study Coordinator
Department of Genetics and Genomic Sciences
Icahn School of Medicine at Mount Sinai
One Gustave L. Levy Place, Box 1498
New York, NY 10029
Tele: 212-659-1427
brittany.keiser@icahn.mssm.edu

The Assistance Fund’s ASMD financial assistance program provides financial assistance for the following:
- Prescription drug assistance (copays, deductibles, and coinsurance) on all FDA-approved treatment for ASMD
- Health insurance premiums
- Therapy administration costs
- Disease management (such as prescribing-physician copayments)
- Treatment-related travel costs
- Genetic testing
The Assistance Fund (TAF) helps patients and families facing high medical out-of-pocket costs by providing financial assistance for co-payments, coinsurance, deductibles, and other health-related expenses. Since its founding in 2009, TAF has helped more than 160,000 children and adults in all 50 states, Washington, DC, and Puerto Rico.
Among TAF’s 80 disease programs is the Acid Sphingomyelinase Deficiency (ASMD) Financial Assistance Program. The Acid Sphingomyelinase Deficiency Financial Assistance Program provides financial assistance for out-of-pocket costs associated with all FDA-approved treatment for ASMD, including prescription drugs (copays, deductibles, and coinsurance), health insurance premiums, therapy administration costs, disease management (such as prescribing physician copayments), treatment-related travel costs, and genetic testing.
To be eligible for assistance, patients must be U.S. citizens or permanent residents, meet certain income requirements, have a diagnosis of the disease named in the disease program, have government or private health insurance, and a prescription for an FDA-approved treatment for ASMD. Once a patient is enrolled in a disease program, their coverage lasts the entire calendar year and there is no cap on the amount of assistance in that calendar year.
To learn more, or to apply today, visit enroll.tafcares.org or call (855) 233-0505.
ASMD Financial Assistance Program One-Pager
The Assistance Fund – Program Reenrollment for 2024
The Assistance Fund Press Release 01/16/2023

